Hearing Wrap Up: FDA’s Food Safety Director Acknowledges ‘Less Than Ideal’ Slow Response to Formula Crisis
WASHINGTON—Today, the Subcommittee on Health Care and Financial Services held a hearing titled “FDA Oversight Part II: Responsibility for the Infant Formula Shortage.” At the hearing, members asked Dr. Susan Mayne, Director of the Center for Food Safety and Applied Nutrition, questions about the Food and Drug Administration’s (FDA) response to the nationwide infant formula shortage, its failure to heed whistleblower warnings, and its failure to conduct a forthright and independent review of the crisis. The hearing furthers the committee’s investigation into the crisis to ensure agencies are held accountable and to find solutions to better protect American families in the future.
Key Takeaways:
The FDA’s failure to prepare for a crisis and slow responsiveness to the crisis had serious implications.
- The FDA ignored a whistleblower report of a Cronobacter sakazakii bacteria outbreak in Abbott Nutrition’s powdered infant formula production for months. Former FDA officials previously testified before the Oversight Committee that the FDA’s failure to conduct an official inspection for four months worsened the crisis.
The Biden Administration was made aware of the formula shortage for months before it took any action.
- Out of stock rates for infant formula in most states hit 74 percent by May 2022. Only then did the Biden Administration take decisive action to ameliorate the crisis. By then, the damage was done, and it took months to restore access to formula.
- “…I am aware that we did send a communication to the supply chain task force, which would include the White House, on February 16th, which was the day before the recall,” Dr. Mayne said during the hearing. However, President Biden has publicly stated that he was not aware of the infant formula shortage until April–months later.
The FDA said previously that nobody has seen the whistleblower report. Today, Dr. Mayne acknowledged that the whistleblower report was received, and was not escalated to senior leadership.
- “There was no process within FDA to escalate this particular whistleblower complaint. FDA receives a large number of whistleblower complaints, so it was a failure of escalation. I do wish I had been made aware of this particular whistleblower complaint,” Dr. Mayne testified during the hearing.
Member Highlights:
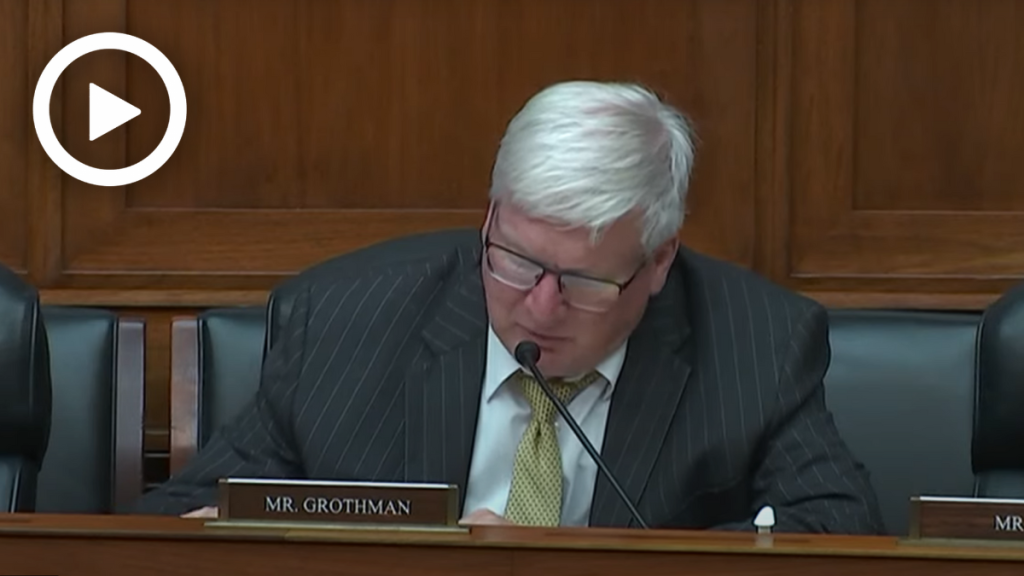
Rep. Glenn Grothman (R-Wis.) asked about the FDA’s decentralized food program structure, which FDA officials have stated on the record prioritizes the drug program over the food program.
Rep. Grothman: “Do you think the FDA is doing enough to ensure food safety?”
Dr. Mayne: “We can always do more to ensure food safety.”
Rep. Grothman: “[The Reagan-Udall Foundation] report describes the FDA’s food division in a culture of constant turmoil. Do you agree with the Reagan-Udall Foundation’s assessment?”
Dr. Mayne: “My indication of their assessment was that the reporting and decision-making needed to be streamlined, which is something that I agree with, and that organizational changes could make a more efficient or effective program, which I also agree with.”
Rep. Grothman also warned that the FDA used the pandemic as a scapegoat for conducting inadequate inspections and ignoring a whistleblower report of a Cronobacter sakazakii.
Rep. Grothman: “COVID-19 was used as an excuse for FDA not receiving a whistleblower complaint about the Abbott plant. Commissioner Califf testified before Congress that the whistleblower report was not transmitted to key FDA food safety officials, like yourself, because of a COVID-19 mailroom issue. Do you believe the report was actually lost in the mail?”
Dr. Mayne: “I can’t speak as to what happened to the report itself being lost in the mail, but what I can speak to is the report was received at FDA–it was received by our field inspection of force, and they worked on the report. They acknowledged that they received the report. […] So, it was not that FDA didn’t act upon it, but that simply senior leadership was not aware of it.”
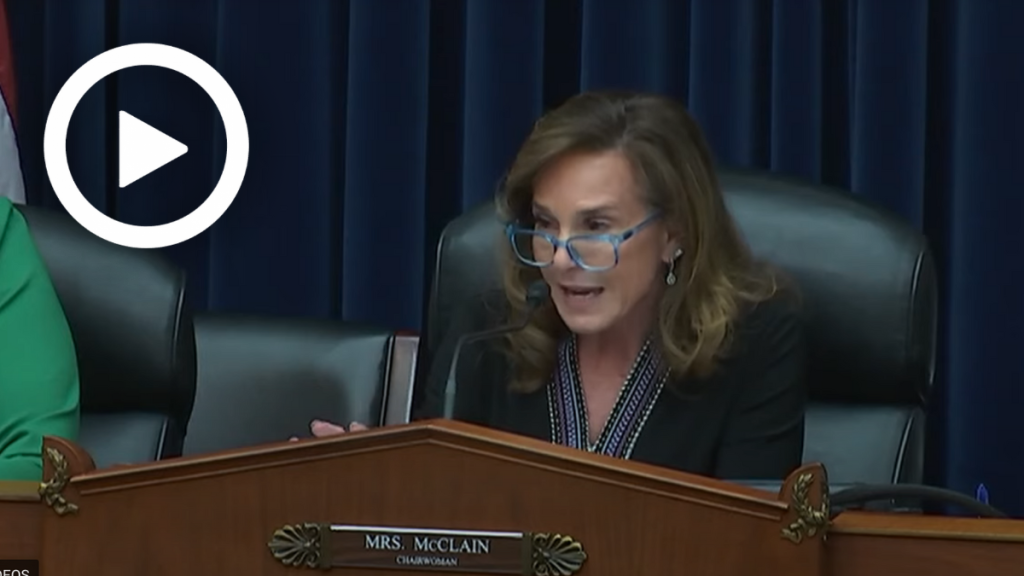
Subcommittee Chairwoman Lisa McClain (R-Mich.) warned that despite the first whistleblower complaint in February 2021, the FDA did take any action until September 2021. Further, four months went by between when the first Cronobacter case was reported to the FDA by the Minnesota Department of Health in September 2021 and the FDA’s inspection in January 2022.
Rep. McClain: “When you went in there at the original September of ’21, that was a normal, regular scheduled visit so to speak. Right?”
Dr. Mayne: “Right.”
Rep. McClain: “Then after that is unfortunately when some babies passed away and there were more complaints right between September and January. Why the four-month lag?”
Dr. Mayne: “We agree with you. Those timelines are less than ideal. And that’s why we have put in place a whole serious of processes based upon the lessons learned from this.”
Rep. Nick Langworthy (R-N.Y.) asked why the Biden Administration delayed taking action to respond to the formula shortage.
Rep. Langworthy: “A week before the formula recall on February 10th, FDA leadership activated the coordinated outbreak response and CORE Network. To your knowledge, were senior White House officials notified at that point?”
Dr. Mayne: “I know that White House officials were notified on February 16th. I don’t know if they were notified on February 10th. But I am aware that we did send a communication to the supply chain task force, which would include the White House, on February 16th, which was the day before the recall.”
Rep. Langworthy: “If the White House was aware, why did it take three months for them to step in and help address the shortage of baby formula?”
CLICK HERE to watch the hearing.
READ MORE: Hearing Wrap Up: Former FDA Official: Infant Formula Crisis Was “A Preventable Tragedy”